Molecular Biology of Methylenetetrahydrofolate-Reductase (MTHF-R) and Overview of the Mutation
 Have you ever looked around an airport and thought to yourself: wow, we all look so different. It’s funny because, when you look a little deeper, you may be surprised how similar we all are despite the difference in our appearances. All humans have basically the same set of genes. Our differences come from tiny variations in these genes, known as Human Genetic Variation. There are multiple variants of any given gene in the human population, however on average, in terms of DNA sequence all humans are 99.8% similar to any other humans. So how is it that we look so different, act so differently and respond so differently to stimuli, both external and internal?
Have you ever looked around an airport and thought to yourself: wow, we all look so different. It’s funny because, when you look a little deeper, you may be surprised how similar we all are despite the difference in our appearances. All humans have basically the same set of genes. Our differences come from tiny variations in these genes, known as Human Genetic Variation. There are multiple variants of any given gene in the human population, however on average, in terms of DNA sequence all humans are 99.8% similar to any other humans. So how is it that we look so different, act so differently and respond so differently to stimuli, both external and internal?
 Although our unique individualities steams from less than 0.2% difference in our DNA sequencing, this minuscule difference has a significant impact in our biological makeup.
Although our unique individualities steams from less than 0.2% difference in our DNA sequencing, this minuscule difference has a significant impact in our biological makeup.
These variations in our genes not only influence how we behave and look, but in how our bodies react differently to external factors. One of these gene variations is seen in the methylenetetrahydrofolate reductase gene, specifically in the 677 and 1298 positions of Chromosome 1. Too much? Well turn up your focus for a moment, this ends up being pretty important. There are some technical parts, but I’ll try to keep it digestible.
An Overview
 Methylenetetrahydrofolate-Reductase (MTHF-R) is a critical regulatory enzyme in folate and homocysteine (an amino acid and breakdown product of protein metabolism). MTHF-R mutation has been linked to an increase risk of heart attacks, strokes and contributes to plaque formation within arterial walls, metabolism, and how your body reacts to the foods you eat and lifestyle you live. For example, someone that has an MTHF-R mutation may have difficulty metabolizing caffeine and/or alcohol and may react differently to them than a person without the mutation.
Methylenetetrahydrofolate-Reductase (MTHF-R) is a critical regulatory enzyme in folate and homocysteine (an amino acid and breakdown product of protein metabolism). MTHF-R mutation has been linked to an increase risk of heart attacks, strokes and contributes to plaque formation within arterial walls, metabolism, and how your body reacts to the foods you eat and lifestyle you live. For example, someone that has an MTHF-R mutation may have difficulty metabolizing caffeine and/or alcohol and may react differently to them than a person without the mutation.
 The involvement of MTHF-R in disease was first published by Mudd et al who identified a patient with homocystinuria (elevated homocysteine) due to a severe deficiency of the enzyme. This severe form of the deficiency is still relatively rare, however a milder form of deficiency is common and can contribute a mild to moderate elevation of plasma and total homocysteine, leading to cardiovascular disease and dysregulation of neurotransmitters in the brain. The two variations of MTHF-R mutation discussed in this article, the A1298C and C677T mutations, have been linked to conditions such as anxiety, migraines, fibromyalgia, ADD/ADHD, dementia, deep vein thrombosis, heart disease, elevated blood pressure, peripheral neuropathy, elevated liver enzymes, elevated cholesterol, infertility and miscarriages, to name a few. It is quite surprising and unique that a single variant should influence such a wide variety of clinical conditions. However given the critical role of folates in DNA synthesis and repair, in homocysteine regulation, and in the methylation cycle, it is understandable why the interest curve in MTHF-R is currently at 250 publications per year.
The involvement of MTHF-R in disease was first published by Mudd et al who identified a patient with homocystinuria (elevated homocysteine) due to a severe deficiency of the enzyme. This severe form of the deficiency is still relatively rare, however a milder form of deficiency is common and can contribute a mild to moderate elevation of plasma and total homocysteine, leading to cardiovascular disease and dysregulation of neurotransmitters in the brain. The two variations of MTHF-R mutation discussed in this article, the A1298C and C677T mutations, have been linked to conditions such as anxiety, migraines, fibromyalgia, ADD/ADHD, dementia, deep vein thrombosis, heart disease, elevated blood pressure, peripheral neuropathy, elevated liver enzymes, elevated cholesterol, infertility and miscarriages, to name a few. It is quite surprising and unique that a single variant should influence such a wide variety of clinical conditions. However given the critical role of folates in DNA synthesis and repair, in homocysteine regulation, and in the methylation cycle, it is understandable why the interest curve in MTHF-R is currently at 250 publications per year.
Enzyme Activity
Note: Mutation, polymorphism, variant and sequence change – These terms are used interchangeably throughout this article to denote the 677CàT and 1298AàC nucleotide substitution.
 Currently, a total of 34 rare but deleterious mutations in MTHF-R, as well as a total of 9 common variants (polymorphisms) have been reported. The enzyme is coded by the gene with the symbol MTHF-R on chromosome 1 at the position 36.3 in humans. The mutated gene can be inherited by a child from both of his/her parents. An individual is said to be heterozygous with MTHF-R mutation if he or she receives one copy of the mutated gene and another copy of the normal gene from his/her parents (A0 or C0). If the individual has two copies of the same mutated gene, he/she said to be homozygous to MTHF-R mutation (AA or CC). If the individual has one copy of each mutation (AC) he/she is said to have double heterozygous mutation. This mutation refers to the inability of MTHF-R gene to activate folic acid to its active and usable form, 5-MTHF. If an individual has a heterozygous mutation, this process is delayed or is impeded by 30% and if the individual has a homozygous or double heterozygous mutation, the activation of folic acid is impeded by roughly 50%. Since Methylation pathways affect over 100 biochemical pathways in the body, it is easy to see why this mutation has such a significant role in our overall health.
Currently, a total of 34 rare but deleterious mutations in MTHF-R, as well as a total of 9 common variants (polymorphisms) have been reported. The enzyme is coded by the gene with the symbol MTHF-R on chromosome 1 at the position 36.3 in humans. The mutated gene can be inherited by a child from both of his/her parents. An individual is said to be heterozygous with MTHF-R mutation if he or she receives one copy of the mutated gene and another copy of the normal gene from his/her parents (A0 or C0). If the individual has two copies of the same mutated gene, he/she said to be homozygous to MTHF-R mutation (AA or CC). If the individual has one copy of each mutation (AC) he/she is said to have double heterozygous mutation. This mutation refers to the inability of MTHF-R gene to activate folic acid to its active and usable form, 5-MTHF. If an individual has a heterozygous mutation, this process is delayed or is impeded by 30% and if the individual has a homozygous or double heterozygous mutation, the activation of folic acid is impeded by roughly 50%. Since Methylation pathways affect over 100 biochemical pathways in the body, it is easy to see why this mutation has such a significant role in our overall health.
MTHF and Homocystein
 The 677CàT (A222V) variant has been particularly noteworthy since it has become recognized as the most common genetic cause of hyperhomocysteinemia. In this mutation, the nucleotide thymine replaces the nucleotide cytosine at position 677 of the MTHF-R gene. In the 1298AàC mutation, the nucleotide cytosine replaces the nucleotide adenine at position 1298 of the MTHF-R gene. Both of these mutations yield a different sequence of amino acids and therefore a different protein to be produced by the body that is less active than the normal enzyme.
The 677CàT (A222V) variant has been particularly noteworthy since it has become recognized as the most common genetic cause of hyperhomocysteinemia. In this mutation, the nucleotide thymine replaces the nucleotide cytosine at position 677 of the MTHF-R gene. In the 1298AàC mutation, the nucleotide cytosine replaces the nucleotide adenine at position 1298 of the MTHF-R gene. Both of these mutations yield a different sequence of amino acids and therefore a different protein to be produced by the body that is less active than the normal enzyme.
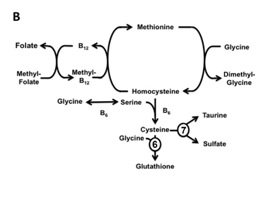
5-Methyltetrahydrofolate is involved in the re-methylation (recycling) of homocysteine to methionine, which is then converted to S-adenosylmethionine (SAMe), the predominant methyl donor in humans. The decreased levels of methionine and SAMe are thought to be an important cause of the neurological problems. Homocysteine can also go through transsulfuration pathway to become glutathione. However this pathway is also dependent on active vitamin B12 and vitamin B6, which are also affected by MTHF-R mutation.
As illustrated in figure B, MTHF is required for the activation of B12 from Cyanocobalamin to its active form Methylcobalamin (Methyl-B12). Without this active form of B12, homocysteine cannot fully recycle back to Methionine nor go down the transsulfuration pathway to become glutathione.
Homocystein Metabolism and the Role of Glutathione
Homocystein is a sulfur containing amino acid whose metabolism is at the intersection of two metabolic pathways: remethylation and transsulfuration. In re-methylation, homocysteine acquires a methyl group from 5-methyltetrahydrofolate to form methionine. This reaction is catalyzed by a methyltransferase (methionine synthase), which requires vitamin B12 as a cofactor. In the transsulfuration pathway,
Glutathione serves as one of the most important anti-oxidants and detoxifiers in the body. It protects our cells from oxidative stress and from mercury and other toxic methyl by transporting them out of the cells and eventually out of the body. Glutathione is also vital to mitochondrial function and energy production as well as one of the few things than can cross the blood brain barrier (BBB). This is why decreased levels of glutathione have been associated with neurodegenerative diseases such as Alzheimer’s, Parkinson’s and Hutington’s disease to name a few. However its impact on our health does not stop there. Glutathione deficiency can also impact liver, chronic age related diseases such as cataracts, immune diseases such as autoimmune diseases and HIV, as well as pulmonary disease like asthma, COPD and bronchitis.
Neurodegenerative and Neuropsychiatric Disease and MTHF-R
In terms of neurodegenerative and neuropsychiatric diseases, the role of MTHF-R mutation may not specifically relate to the pathogenesis of the disease, but may be a factor that renders a person vulnerable and thus triggers the disease or precipitates its course. Most of the studies have investigated the role of MTHF and depression. Folate deficiency or inability to activate folic acid to methyl folate has been associated with a wide range of abnormal mental states, and particularly with affective disorders. One possible explanation is that methyl folate plays an important role in production of tetrahydrobiopterin (BH4), an essential cofactor for tryptophan hydroxylase in serotonin synthesis, and for tyrosine hydroxylase in dopamine synthesis.
Tryptophan hydroxylase (TPH) is an enzyme involved in the synthesis of the neurotransmitter serotonin. It is also the first enzyme in the synthesis of melatonin.
 Individuals with low active folate levels (MTHF) are more likely to suffer from the most severe forms of depression and are significantly less likely to respond to treatment with anti-depressants. Furthermore, in a recent study, 52% of inpatients with severe depression had raised plasma homocysteine levels. In a double-blind placebo-controlled clinical trial, Godfrey et al added 15mg of methylfolate to standard psychotropic medication and reported significant and increasing clinical and social recovery of patient in a six month period. Not having sufficient levels of methylfolate in the brain can have a significant negative impact on the brain’s ability to manufacture serotonin and dopamine. Mutations in MTHF-R gene, in particular A1298C variant can interfere with the recycling of Tetrahydrobiopterin (BH4); the cofactor needed to produce dopamine and serotonin and lead to less severe, but still clinically significant deficiency of BH4. The reason that activated folate is so important for depression is due to the fact that these vitamins increase BH4 levels. The more BH4 we make the more our brain is able to convert tryptophan into serotonin. Adequate levels of serotonin means less depression. Of course there is more to it than this one reaction, however this is one of the reasons why the MTHF-R gene impacts depression. As our body struggles to activate folate through the impeded MTHF-R pathway, we produce less BH4, leading to a deficiency in dopamine and serotonin. In order to overcome depression, one must figure out which neurotransmitters are in excess and which are deficient. To fully understand this complex relationship and the role BH4 plays we have to dig deep in the brain chemistry which gets a bit complicated, even for this article.
Individuals with low active folate levels (MTHF) are more likely to suffer from the most severe forms of depression and are significantly less likely to respond to treatment with anti-depressants. Furthermore, in a recent study, 52% of inpatients with severe depression had raised plasma homocysteine levels. In a double-blind placebo-controlled clinical trial, Godfrey et al added 15mg of methylfolate to standard psychotropic medication and reported significant and increasing clinical and social recovery of patient in a six month period. Not having sufficient levels of methylfolate in the brain can have a significant negative impact on the brain’s ability to manufacture serotonin and dopamine. Mutations in MTHF-R gene, in particular A1298C variant can interfere with the recycling of Tetrahydrobiopterin (BH4); the cofactor needed to produce dopamine and serotonin and lead to less severe, but still clinically significant deficiency of BH4. The reason that activated folate is so important for depression is due to the fact that these vitamins increase BH4 levels. The more BH4 we make the more our brain is able to convert tryptophan into serotonin. Adequate levels of serotonin means less depression. Of course there is more to it than this one reaction, however this is one of the reasons why the MTHF-R gene impacts depression. As our body struggles to activate folate through the impeded MTHF-R pathway, we produce less BH4, leading to a deficiency in dopamine and serotonin. In order to overcome depression, one must figure out which neurotransmitters are in excess and which are deficient. To fully understand this complex relationship and the role BH4 plays we have to dig deep in the brain chemistry which gets a bit complicated, even for this article.
Conclusion
The association between methylfolate deficiency and the generation of abnormal DNA methylation patterns is a relatively recent discovery. Continued research into this new and provocative relationship has the potential to provide new insight into the expression and progression of several diseases, neurological disorders, aging and even cancers. There are numerous mutations in MTHF-R that cause reduction in the enzyme activity. In general, the more severely reduced the enzyme activity is, the more pathways it can affect. This leads to a more complex clinical picture. Clinical presentation or symptoms can occur any time from the neonatal period to adulthood. There are also causes that are asymptomatic. In such cases, the determents of MTHF-R mutation can be seen in blood work such as elevated liver enzymes, elevated cholesterol, elevated cortisol levels and/or decrease thyroid activation. Overall methylation plays an essential role in our everyday health and a delayed activation or methylation process can be a crucial factor in delayed overall recovery or treatment processes.
Sources
- American Society of Human Genetics / American College of Medical Genetics Test and technology Trafer Committee Working Group. ASHG / ACMG statement. Measurement and use of total plasma homocysteine. Am J Hum Genet 1998; 63:1541-1543
- Landes Bioscience. Medical Intelligence Unit. MTHFR polymorphisms and Disease. Molecular Biology of Methylenetetrahydrofolate Reductase and Overview of Mutations/Polymorphisms 2005; 1:1-20
- Medical Intelligence Unit. Severe Methylenetetrahydrofolate Reductase Deficiency. Mary A Thomas, David S. Rosenblatt. MTHFR Polymorphisms ad Disease 2005; 4:41-53
- Medical Intelligence Unit. Methylenetetrhydrofolate Reductase and Venous Thrombosis. Miranda B.B.J. Keijzer, Martin den Heijer. MTHFR Polymorphisms ad Disease 2005; 9:113-124
- Medical Intelligence Unit. Mild MTHFR Deficiency and Folate Status. Paul F Jacques, Silvina F. Choumenkovitch. MTHFR Polymorphisms ad Disease 2005; 5:54-71
- Medical Intelligence Unit. Neuropsychiatric Disease and Methylenetetrahydrofolate Reductase. Bjorn Regland. MTHFR Polymorphisms ad Disease 2005; 12:163-69
- Bottiglieri T, Laundy M, Crellin R et al. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry 2000; 69:228-32
- Godfrey PSA, Toone BK, Carney MWP et al. Enhancement of revery from psychiatric illness by methylfolate. Lancet 1990; 336:392-95
